Medical Mattress: Pressure Sore Prevention & Optimal Distribution
Understanding Advanced Pressure Relief Technologies in Medical Mattresses
In the evolving landscape of healthcare, the role of specialized bedding solutions has become increasingly critical, particularly for patients facing prolonged immobility. A high-performance medical mattress is no longer merely a comfort accessory but a fundamental therapeutic tool designed to prevent and manage pressure injuries, commonly known as bedsores or decubitus ulcers. These debilitating conditions afflict millions globally, leading to extended hospital stays, increased healthcare costs, and severe patient discomfort. The escalating global aging population, coupled with a rise in chronic diseases, intensifies the demand for effective preventative measures. Modern pressure relief technologies, such as advanced pressure distribution mattress systems, are engineered to meticulously redistribute body weight, minimize localized pressure points, and mitigate shear forces, which are pivotal factors in skin breakdown. This proactive approach to patient care underscores a critical shift from reactive treatment to preventative interventions, safeguarding patient well-being while simultaneously optimizing resource allocation within healthcare facilities. The development of such mattresses represents a confluence of biomechanical understanding, material science innovation, and clinical efficacy, directly addressing a pervasive challenge in contemporary medical practice.
Current industry trends in pressure relief mattress technology reflect a profound commitment to patient safety, operational efficiency, and long-term sustainability. The market is witnessing a surge in modular designs that allow for easy replacement of individual components, extending product lifespan and reducing waste. Innovations extend to the incorporation of "smart" features, such as integrated sensors for continuous pressure monitoring and alarm systems that alert caregivers to high-risk areas, enhancing the proactive capabilities of a medical mattress. Furthermore, there's a heightened focus on material advancements, including breathable fabrics with superior moisture vapor transmission rates (MVTR) to manage skin microclimate and antimicrobial treatments to reduce infection risks. From an economic perspective, preventing even one Stage III or IV pressure ulcer can save healthcare institutions tens of thousands of dollars in treatment costs, making investments in advanced pressure sore prevention mattress solutions a financially prudent decision. The drive towards evidence-based practice further compels manufacturers to provide robust clinical data demonstrating the efficacy of their products in real-world scenarios, ensuring that healthcare providers can make informed procurement decisions that genuinely improve patient outcomes and operational sustainability. These trends collectively underscore the sophisticated evolution of the medical mattress from a simple bedding item to a complex, technologically advanced medical device.
The Core Manufacturing Process of a High-Performance Medical Mattress
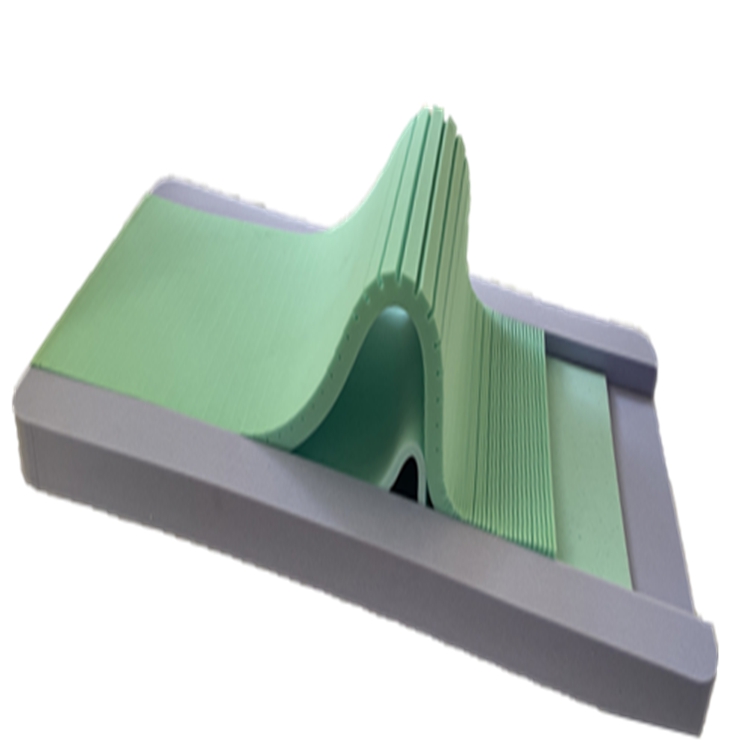
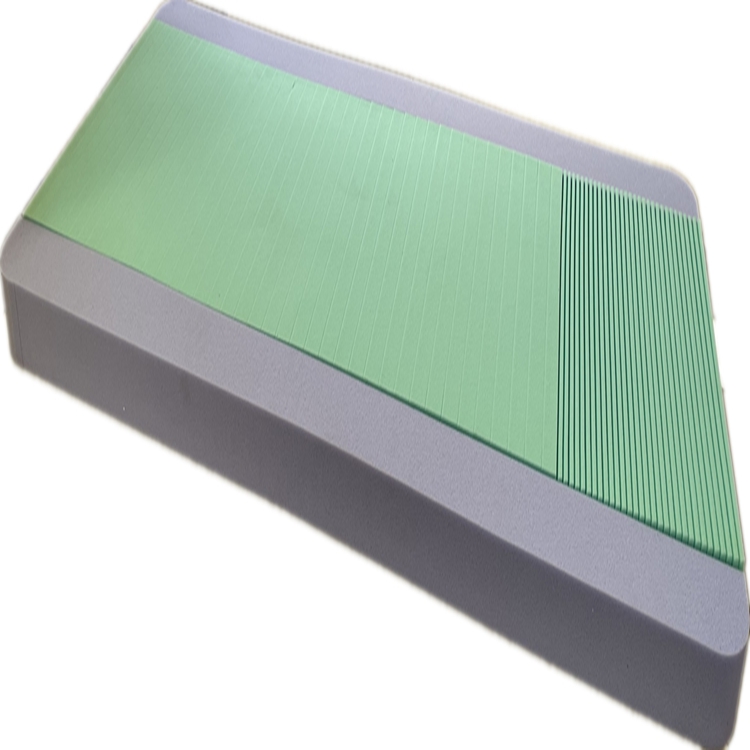
The production of a high-performance medical mattress, such as the Three-layer Pressure-Relief Mattress, involves a meticulous, multi-stage manufacturing process designed to ensure unparalleled efficacy and durability. The foundational step involves the careful selection of premium raw materials, including various grades of polyurethane foams (e.g., high-resiliency foam, viscoelastic memory foam, and often a firm base foam), and specialized cover fabrics. Each foam layer is chosen for its specific properties: the top layer for optimal pressure redistribution and comfort, the middle layer for progressive support and conforming capabilities, and the base layer for stability and bottom-out prevention. Rigorous quality control checks are performed on incoming materials to verify density, Indentation Load Deflection (ILD), and overall structural integrity, ensuring consistency and performance. The manufacturing then proceeds to precision cutting using advanced CNC (Computer Numerical Control) machinery, which ensures accurate dimensions and contours for each foam layer, minimizing material waste and maximizing the therapeutic design. Following cutting, a sophisticated lamination process bonds the distinct foam layers together, often using solvent-free, medical-grade adhesives to create a cohesive unit that optimizes pressure redistribution and provides dynamic support across the mattress surface.
Post-lamination, the mattress core undergoes detailed shaping and contouring, particularly for models featuring specialized zones or channels designed to enhance airflow or provide targeted pressure relief. Concurrently, the specialized covers are fabricated from high-performance materials like fluid-proof, breathable, vapor-permeable, and sometimes antimicrobial-treated nylon or polyurethane-coated fabrics. These covers are precisely cut and meticulously sewn using industrial-grade stitching to create a durable, protective barrier that is easy to clean and disinfect, crucial for infection control in clinical environments. Throughout the assembly process, adherence to stringent quality management systems, notably ISO 13485, is paramount. This international standard specifies requirements for a comprehensive quality management system for the design and manufacture of medical devices, ensuring that every stage, from material procurement to final assembly, meets the highest benchmarks for safety and performance. Regular in-process inspections verify dimensional accuracy, layer adhesion, and seam integrity. This meticulous attention to detail ensures that the final pressure distribution mattress not only performs as designed but also withstands the rigorous demands of continuous clinical use, extending its service life and providing consistent therapeutic benefits.
The final stages of manufacturing encompass comprehensive testing, packaging, and regulatory compliance. Each completed medical mattress undergoes rigorous performance verification, including simulated pressure mapping tests to confirm its pressure redistribution capabilities and durability testing to assess its longevity under various load conditions. These tests often comply with international standards to validate product claims regarding pressure relief, shear reduction, and overall structural integrity. Once certified, the mattresses are meticulously packaged to protect them during transit, ensuring they arrive in pristine condition. Furthermore, obtaining and maintaining critical regulatory approvals such as FDA registration for the United States market and CE Marking for the European Union is a non-negotiable step. These certifications signify that the product meets the stringent health, safety, and environmental protection standards required for medical devices in these regions. The entire manufacturing lifecycle, from initial design concepts and material sourcing through to final testing and distribution, is orchestrated with precision, guaranteeing that the end product delivers consistent, reliable pressure sore prevention and contributes significantly to patient recovery and comfort across diverse healthcare settings. This robust process underscores the commitment to delivering medical-grade quality and reliability.
Key Technical Specifications and Performance Metrics for Medical Mattresses
The technical prowess of a sophisticated medical mattress is quantified by a range of critical specifications and performance metrics that directly impact its therapeutic efficacy and longevity. Material composition is foundational, typically involving multi-layered polyurethane foams with varying densities and firmness levels (measured by Indentation Load Deflection or ILD/IFD). For instance, a common configuration might feature a top layer of softer, low-ILD viscoelastic foam for immersion and envelopment, followed by a medium-firm high-resiliency foam for transitional support, and a very firm base foam to prevent bottoming out. Density (e.g., 1.8-2.5 lbs/ft³ for support foams, 3.0-5.0 lbs/ft³ for memory foam) indicates material longevity and load-bearing capacity. Crucially, the ability of the mattress to reduce interface pressure, often quantified through pressure mapping systems, is paramount; a high-performing pressure distribution mattress aims to keep peak pressures below 32 mmHg, which is the generally accepted capillary occlusion pressure. Beyond pressure, shear force reduction is vital, addressed by materials that allow for slight movement between layers, preventing skin tearing as a patient repositions or is moved. Furthermore, the mattress cover's breathability, measured by Moisture Vapor Transmission Rate (MVTR), plays a significant role in maintaining optimal skin microclimate, preventing maceration, and thereby reducing the risk of pressure injury development.
| Parameter | Specification | Benefit |
|---|---|---|
| Mattress Type | Three-layer Foam Pressure Relief | Optimized support, comfort, and pressure redistribution |
| Overall Dimensions | 80" L x 36" W x 6" H (Standard) | Fits most standard hospital beds; customizable sizes available |
| Top Layer Material | 2" Viscoelastic Memory Foam (5.0 lbs/ft³ density, 10 ILD) | Superior immersion, envelopment, and pressure peak reduction |
| Middle Layer Material | 2" High-Resiliency Foam (2.2 lbs/ft³ density, 30 ILD) | Dynamic support, comfort, and shear reduction |
| Base Layer Material | 2" High-Density Base Foam (1.8 lbs/ft³ density, 45 ILD) | Prevents bottoming out, provides stable foundation |
| Cover Material | Polyurethane-Coated Nylon, Fluid-Proof, Vapor Permeable | Infection control, moisture management, easy cleaning |
| Weight Capacity | Up to 500 lbs (227 kg) | Accommodates a broad range of patient weights |
| Flame Retardancy | Meets Cal 117, 16 CFR 1632, 16 CFR 1633 (optional) | Ensures patient safety in case of fire |
| Service Life Expectancy | 7-10 years (with proper care) | Long-term cost-effectiveness for healthcare facilities |
Beyond material properties, a truly effective pressure sore prevention mattress must exhibit superior dynamic performance characteristics. For instance, its ability to maintain consistent pressure redistribution regardless of patient movement or repositioning is crucial. This is often demonstrated through sustained pressure mapping tests over several hours, indicating the mattress's capacity to prevent localized pressure build-up. The mattress's envelopment properties, which describe how well it conforms to the body's contours, are essential for maximizing the body's surface area in contact with the mattress, thus distributing pressure more broadly. Further technical considerations include the mattress's resistance to compression set, which measures its ability to return to its original shape after prolonged loading, directly influencing its long-term durability and therapeutic effectiveness. The cover's friction coefficient and shear properties are also critical, as high friction or shear can damage fragile skin. Advanced covers are designed to reduce these forces, providing a low-friction interface. Comprehensive flame retardancy ratings, such as compliance with Cal 117 or even stricter standards like 16 CFR 1633, are non-negotiable for safety. The culmination of these technical specifications ensures that a specialized medical mattress delivers consistent, measurable therapeutic benefits, making it an indispensable asset in modern patient care.
Application Scenarios and Strategic Advantages of Specialized Pressure Relief Mattresses
Specialized pressure relief mattresses are indispensable across a broad spectrum of healthcare environments, each with unique patient populations and clinical demands. In acute care hospitals, they are essential for post-operative patients, those in critical care units, or individuals with limited mobility due to trauma or neurological conditions. These settings require a medical mattress that can rapidly adapt to changing patient needs and provide immediate, effective pressure redistribution. Long-term care facilities, including nursing homes and rehabilitation centers, constitute another primary application area, catering to elderly residents or individuals requiring extended rehabilitation. Here, the focus shifts to long-term comfort, durability, and robust pressure sore prevention for patients who may spend the majority of their day in bed. Home healthcare settings also increasingly utilize these advanced mattresses for patients transitioning from hospital care or those with chronic conditions managed at home, emphasizing ease of maintenance and integration into a non-clinical environment. Typical patient populations benefiting from these mattresses include individuals with spinal cord injuries, cerebral palsy, muscular dystrophy, severe obesity, or those undergoing palliative care, all of whom are at heightened risk of pressure ulcer development due to impaired sensation, limited mobility, or poor nutritional status.
The strategic advantages of deploying a high-quality pressure distribution mattress extend far beyond mere patient comfort, encompassing significant clinical and operational benefits for healthcare providers. Clinically, the primary advantage is the dramatic reduction in the incidence and severity of pressure injuries. By effectively redistributing pressure, managing shear forces, and controlling microclimate, these mattresses proactively prevent the painful and often life-threatening complications associated with bedsores. This translates into improved patient outcomes, faster recovery times, and enhanced overall quality of life. Operationally, the cost savings are substantial. Treating a single Stage IV pressure ulcer can cost upwards of $70,000, not including the associated litigation risks and negative institutional reputation. By investing in advanced pressure sore prevention mattress technology, hospitals and care facilities can significantly reduce these direct and indirect costs, reallocating resources to other critical areas of patient care. Furthermore, these mattresses can reduce nursing workload by minimizing the frequency of patient repositioning required for pressure relief. Advanced fluid-proof and easy-to-clean covers also contribute to superior infection control protocols, an increasingly vital consideration in today’s healthcare environment. The long service life and durability of these specialized mattresses further enhance their value proposition, offering a robust and cost-effective solution for comprehensive patient well-being and institutional efficiency.
Navigating Manufacturer Choices and Customization Solutions in Medical Mattress Procurement
For B2B decision-makers and technical procurement specialists, selecting the right manufacturer for a medical mattress is a critical decision that influences patient safety, operational costs, and long-term institutional reputation. Key factors to consider include the manufacturer's R&D capabilities, indicated by their commitment to continuous innovation in pressure relief technology and their portfolio of patented designs. Certifications are non-negotiable: look for ISO 9001 for general quality management and, more importantly, ISO 13485 for medical device quality management systems, ensuring stringent adherence to regulatory standards throughout the entire production lifecycle. A manufacturer's production capacity and supply chain reliability are also crucial, particularly for large-scale orders or facilities requiring consistent, timely replenishment. Evaluating past performance through client testimonials, case studies, and industry awards provides valuable insight into a manufacturer's proven track record. Furthermore, assessing the robustness of their customer support and after-sales service, including technical assistance and expedited spare parts availability, is vital for ensuring seamless integration and ongoing maintenance of the pressure distribution mattress within your facility. A transparent and reputable manufacturer will readily provide documentation for material sourcing, testing protocols, and compliance with international healthcare standards, fostering a relationship built on trust and mutual objectives.
Beyond standard product offerings, the ability to provide tailored customization solutions is a significant differentiator for leading medical mattress manufacturers. Healthcare environments often have specific spatial constraints, unique bed frame requirements, or particular patient demographics that necessitate non-standard mattress dimensions or configurations. A flexible manufacturer can accommodate varying lengths, widths, and heights to precisely fit specialized beds, such as bariatric beds or pediatric cribs. Customization extends to internal foam architecture; for instance, specific foam types or densities can be adjusted in different zones to address unique pressure points for patients with particular medical conditions, or to optimize bariatric support. Cover materials can also be customized to enhance specific properties, such as a higher MVTR for patients with severe incontinence, or integrated sensor pockets for "smart bed" integration. This level of customization ensures that the procured pressure sore prevention mattress is not merely an off-the-shelf product but a precisely engineered solution optimized for the specific clinical requirements of the client. Engaging with a manufacturer capable of extensive customization allows healthcare providers to optimize clinical effectiveness, improve patient outcomes, and achieve greater operational efficiency by ensuring a perfect fit for every application.
Ensuring Trustworthiness: FAQs, Delivery, and Warranty Commitments
Frequently Asked Questions (FAQs)
-
Q: How does a Three-layer Pressure-Relief Mattress specifically prevent pressure injuries?
A: Our Three-layer Pressure-Relief medical mattress utilizes a sophisticated foam construction where each layer serves a distinct purpose. The top viscoelastic layer conforms precisely to the patient’s body, distributing weight over a larger surface area to minimize peak pressure points. The middle layer provides progressive support, absorbing and mitigating shear forces which are crucial in skin breakdown. The firm base layer prevents bottoming out, ensuring consistent support even during patient movement or extended use. This multi-layered design combined with specialized cover materials actively manages pressure, friction, shear, and microclimate, all critical factors in pressure injury prevention. -
Q: What is the recommended cleaning and maintenance for this medical mattress?
A: The mattress features a fluid-proof, vapor-permeable cover that can be easily cleaned with standard hospital-grade disinfectants and warm water. For routine cleaning, wipe down the surface thoroughly and allow to air dry. For deeper cleaning or disinfection, follow the specific instructions provided with your cleaning agent. It is crucial to avoid harsh abrasives or solvents which could damage the cover material. Regular inspection for any tears or punctures in the cover is also recommended to maintain hygiene and protective properties. Proper care significantly extends the service life and efficacy of your pressure distribution mattress. -
Q: What is the typical service life of your medical mattresses?
A: With proper care and maintenance, our Three-layer Pressure-Relief medical mattress is designed for exceptional durability, offering an expected service life of 7 to 10 years. This extended lifespan is a direct result of using high-quality, resilient foam materials that resist compression set and superior, robust cover fabrics that withstand frequent cleaning and disinfection cycles. Regular rotation (head-to-foot) can also help to ensure even wear and maximize the product's longevity, providing excellent long-term value for healthcare facilities.
Effective logistics and clear delivery protocols are paramount for B2B procurement, ensuring that specialized equipment like a medical mattress reaches its destination on time and in perfect condition. Our streamlined supply chain management system is designed to handle orders of varying scales, from single unit replacements to large-volume institutional procurements. Typical lead times range from 2-4 weeks for standard products, with specific timelines for custom orders provided upon quotation, contingent on material availability and order complexity. We partner with reputable global freight and logistics providers experienced in handling medical equipment, ensuring secure and efficient transit. Each mattress is carefully packaged in robust, protective materials to prevent damage from moisture, dust, or physical impact during shipping. For bulk orders, we offer palletized shipping solutions to optimize transport efficiency and reduce handling risks. Our dedicated logistics team works closely with clients to coordinate delivery schedules, providing tracking information and ensuring seamless integration into your facility's receiving procedures. This commitment to reliable delivery minimizes downtime and supports continuous patient care operations.
Establishing trust in the B2B landscape is built upon clear, comprehensive warranty commitments and robust post-sales support. Our Three-layer Pressure-Relief Mattress comes with a comprehensive warranty package, typically covering manufacturing defects for 7-10 years for the foam core and 2 years for the outer cover, reflecting our confidence in the product's quality and durability. Specific warranty terms and conditions are always provided in detail with each quotation and purchase order. Beyond the warranty period, our commitment extends to continuous customer support, offering technical assistance for product inquiries, maintenance guidance, and troubleshooting. Our dedicated service team is readily available to address any concerns, ensuring optimal performance and longevity of your pressure sore prevention mattress. We also provide access to a comprehensive library of resources, including detailed product manuals, cleaning protocols, and clinical application guides, empowering your staff with the knowledge needed for effective use and care. This holistic approach to customer service, from initial inquiry through years of operation, underscores our dedication to forging lasting partnerships and ensuring complete client satisfaction.
References and Further Reading
- National Pressure Ulcer Advisory Panel (NPUAP). Prevention and Treatment of Pressure Ulcers: Clinical Practice Guideline.
- European Pressure Ulcer Advisory Panel (EPUAP). International Guideline: Prevention and Treatment of Pressure Ulcers.
- Black, J. M., et al. Pressure ulcer epidemiology: a systematic review. Journal of Wound Ostomy & Continence Nursing.
- Gefen, A. The biomechanics of pressure ulceration. Medical Engineering & Physics.
- Edsberg, L. E., et al. Revised National Pressure Ulcer Advisory Panel Pressure Injury Staging System: Updated Definitions and Pictures. Journal of Wound Ostomy & Continence Nursing.
-
Mattresses Designed for Back Pain ReliefNewsAug.08,2025
-
Innovative Wave Mattresses for Ultimate ComfortNewsAug.08,2025
-
High-Quality Mattresses for Hospital BedsNewsAug.08,2025
-
High-Quality Mattresses for Every NeedNewsAug.08,2025
-
Healthcare Foam Mattress: Sleep Better, Heal FasterNewsAug.08,2025
-
Cube Mattress for Daily ComfortNewsAug.08,2025
-
How Hospital Mattress Choices Directly Impact Patient Comfort and CareNewsAug.05,2025

