Medical Mattress: Pressure Sore Prevention & Distribution
The Imperative Role of Advanced Pressure-Relief Mattresses in Modern Healthcare
In contemporary healthcare, the significance of specialized support surfaces, particularly the medical mattress, cannot be overstated. These critical devices are engineered not merely for patient comfort but primarily for the prevention and management of pressure injuries, commonly known as bedsores or decubitus ulcers. With an aging global population and an increasing prevalence of chronic conditions requiring prolonged bed rest, the demand for high-performance pressure distribution mattress solutions is escalating. Industry trends indicate a strong shift towards materials offering superior pressure redistribution, enhanced breathability, and integrated infection control features. This evolution reflects a deeper understanding of biomechanics and patient physiology, aiming to mitigate the complex interplay of pressure, shear, and friction that contributes to skin breakdown.
The economic burden of pressure injuries is substantial, with treatment costs ranging significantly depending on ulcer severity. Beyond the financial impact, these injuries severely diminish patient quality of life, extending hospital stays and increasing mortality rates. Consequently, healthcare providers are increasingly investing in sophisticated pressure sore prevention mattress technologies that offer demonstrable clinical efficacy and long-term durability. Innovations are driving the market towards multi-layered systems, advanced foam compositions, and smart mattress technologies capable of dynamic pressure adjustment. These advancements underscore a proactive approach to patient care, moving beyond reactive treatment to preventative strategies that safeguard patient well-being and optimize healthcare outcomes.
Precision Engineering: The Manufacturing Process of a Medical Mattress
The production of a high-quality medical mattress like the Three-layer Pressure-Relief Mattress is a meticulously controlled process, adhering to stringent quality and safety standards. It begins with the selection of premium materials, typically medical-grade foams and specialized cover fabrics. For pressure-relief mattresses, this often includes a combination of visco-elastic (memory) foam, high-resilience foam, and a denser support foam. Each foam type is chosen for its specific properties: memory foam for conformity and pressure redistribution, high-resilience foam for elasticity and responsiveness, and support foam for foundational stability and durability. The specific densities of these foams are crucial parameters, often ranging from 40-80 kg/m³ for visco-elastic layers and 25-35 kg/m³ for base layers, impacting the mattress's Indentation Load Deflection (ILD) and overall feel.
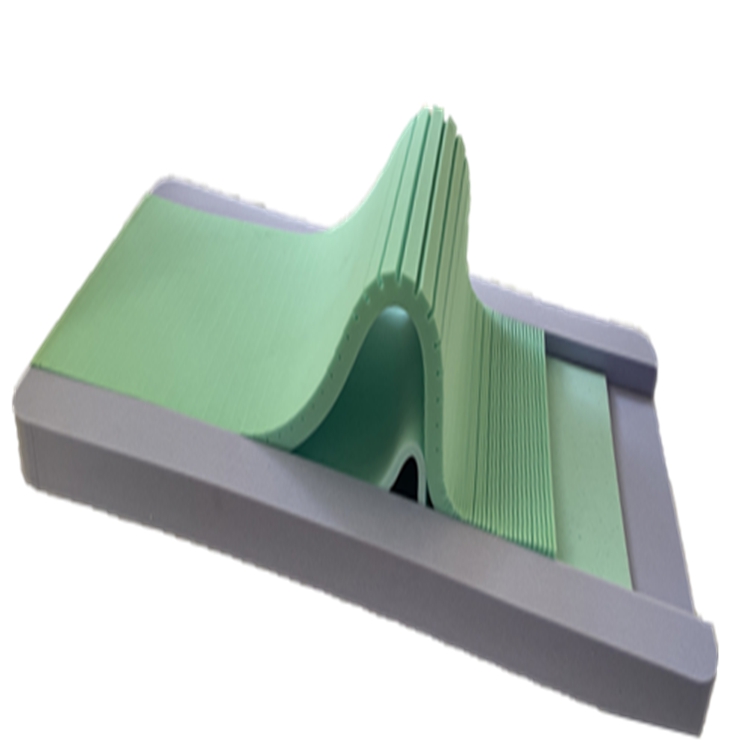
The manufacturing process involves several critical stages. First, large foam blocks are precision-cut using advanced CNC machinery to achieve the exact dimensions and profiles for each layer. This ensures optimal pressure relief zones and anatomical support. Subsequently, these layers are laminated together using medical-grade, solvent-free adhesives, guaranteeing layer integrity and preventing delamination over time. The outer cover, typically made from vapor-permeable, fluid-resistant, and anti-bacterial polyurethane-coated fabric, is then precision-cut and sewn, often incorporating a concealed zipper for easy removal and cleaning. The final assembly involves encapsulating the laminated foam core within this protective cover.
Rigorous quality control and testing are integral at every stage. Products must comply with international standards such as ISO 13485 for Medical Devices Quality Management Systems, ensuring consistent quality and safety. Fire retardancy is verified against standards like CFR 16 Part 1632/1633 (USA) or BS 7177 (UK). Durability testing, including cyclic loading, assesses the mattress's lifespan, often projected at 5-7 years for high-use environments. The mattress's pressure redistribution capabilities are verified using sophisticated pressure mapping systems, ensuring peak pressure points are effectively mitigated. These stringent processes ensure the final product delivers superior performance for applications in hospitals, nursing homes, and home healthcare, providing critical benefits such as enhanced patient comfort, significant reduction in pressure injury incidence, and improved infection control through easy-to-clean surfaces.
Technical Specifications and Performance Metrics for Advanced Medical Mattresses
Understanding the technical specifications of a medical mattress is crucial for procurement and clinical efficacy. Key parameters provide insight into performance, durability, and suitability for various patient needs. For instance, the Three-layer Pressure-Relief Mattress is designed with specific foam densities and compositions to optimize pressure redistribution, minimizing peak pressures and shear forces that contribute to tissue damage. The top layer, often a memory foam, conforms precisely to the patient's body, maximizing contact area and evenly distributing weight. The middle layer typically provides transition and support, while the base layer offers stability and prevents bottoming out.
| Parameter | Description | Typical Value / Standard |
|---|---|---|
| Mattress Type | Static Pressure-Relief, Multi-layered | Three-layer Foam Core |
| Top Layer Material | Visco-elastic (Memory) Foam | Density: 80 kg/m³; ILD: 10-14 |
| Middle Layer Material | High-Resilience Foam | Density: 30 kg/m³; ILD: 25-30 |
| Base Layer Material | High-Density Support Foam | Density: 35 kg/m³; ILD: 35-40 |
| Cover Material | PU-coated Polyester/Nylon | Vapor Permeable, Fluid Resistant, Anti-bacterial |
| Mattress Dimensions (Standard) | Length x Width x Height | 2000 x 900 x 150 mm (Customizable) |
| Maximum Patient Weight | Recommended Weight Capacity | Up to 250 kg (550 lbs) |
| Flammability Standards | Compliance with fire safety regulations | CFR 16 Part 1632 & 1633, BS 7177 |
| Warranty Period | Manufacturer's Guarantee | 5-7 Years (Foam Core) |
Beyond material composition, testing protocols are paramount. Pressure mapping tests, using advanced sensor arrays, demonstrate the mattress's ability to lower interface pressures below capillary closing pressure, a critical factor in preventing pressure injury. Durability tests, such as sustained compression and repeated impact, simulate years of use, confirming the long-term integrity of the foam structure and cover material. Furthermore, the vapor permeability of the cover fabric is measured to ensure effective microclimate management, reducing heat and moisture buildup that can lead to skin maceration. These rigorous evaluations ensure that the medical mattress provides not only immediate comfort but also sustained therapeutic benefits, aligning with the highest standards of patient care and operational efficiency in healthcare settings.
Application Scenarios and Strategic Advantages in Healthcare Environments
The application of a specialized medical mattress extends across various critical healthcare environments, each benefiting from its unique design to support patient recovery and prevent complications. In acute care hospitals, these mattresses are indispensable in intensive care units (ICUs) and general medical-surgical wards, where patients may be immobile for extended periods. Their ability to redistribute pressure effectively reduces the risk of hospital-acquired pressure injuries, a key quality indicator and patient safety concern. Similarly, in long-term care facilities and nursing homes, where residents often have chronic conditions limiting mobility, a high-performance pressure distribution mattress is fundamental to maintaining skin integrity and enhancing overall comfort.
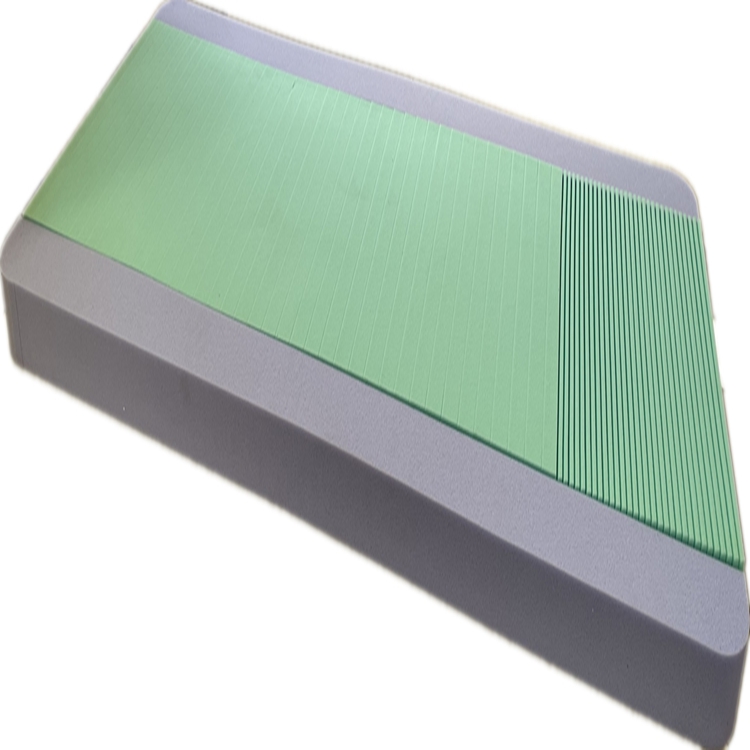
Beyond institutional settings, the demand for sophisticated pressure sore prevention mattress solutions is growing in home healthcare, supporting individuals recovering from surgery or managing chronic illnesses in their own residences. The strategic advantages of investing in an advanced medical mattress are multi-faceted. Clinically, they significantly reduce pressure injury rates, leading to improved patient outcomes, reduced pain, and enhanced quality of life. Operationally, these mattresses minimize the nursing time required for repositioning and wound care, freeing up valuable staff resources. Furthermore, the durable, easy-to-clean, and anti-microbial covers contribute to superior infection control, a paramount concern in all healthcare settings. This holistic benefit profile makes a compelling case for the widespread adoption of such specialized support surfaces.
Navigating the Market: Manufacturer Comparison and Customization Solutions
The market for medical mattress solutions is diverse, ranging from established global leaders to innovative emerging manufacturers. When evaluating potential partners, healthcare facilities should consider several factors beyond initial cost, including a manufacturer's commitment to R&D, adherence to international quality standards (e.g., ISO 9001, ISO 13485, FDA registration where applicable), and proven track record in clinical settings. Leading manufacturers often distinguish themselves through proprietary foam formulations, advanced cover materials, and robust testing methodologies that ensure consistent performance and longevity. Emerging players may offer competitive pricing or specialized niche products but require thorough due diligence regarding their quality assurance processes and post-sales support.
| Factor | Established Global Manufacturers | Specialized Niche / Emerging Manufacturers |
|---|---|---|
| R&D Investment | High, often with dedicated research centers | Moderate to high, focused on specific innovations |
| Certifications & Compliance | ISO 13485, FDA, CE Mark, numerous international standards | ISO 9001, often pursuing specific medical device certifications |
| Product Range | Comprehensive range (foam, air, hybrid) | Specialized in specific types (e.g., foam pressure distribution mattress) |
| Customization Capabilities | High, for large volume orders and specific clinical needs | Agile, often more flexible for bespoke smaller orders |
| Global Presence & Support | Extensive distribution, global service networks | Regional or growing international presence, variable support |
| Clinical Evidence/Studies | Numerous peer-reviewed studies and clinical trials | Growing portfolio of case studies and internal data |
Customization is a pivotal aspect, especially for specialized healthcare needs. A reputable manufacturer of medical mattress products will offer tailored solutions regarding dimensions, foam configurations (e.g., heel cut-outs, specialized zoning), cover materials, and even aesthetic considerations to match specific facility requirements. This flexibility ensures that the pressure sore prevention mattress integrates seamlessly into existing care protocols and equipment, maximizing its therapeutic impact. For instance, the Three-layer Pressure-Relief Mattress can be adapted in terms of thickness, width, and length to fit various bed frames, or incorporate specific features like sloped sections for limb positioning or reinforced edges for patient transfer safety. This bespoke approach facilitates optimal patient care and operational efficiency.
Ensuring Trust: FAQs, Delivery, and Warranty Commitments
Building trust in the B2B healthcare sector hinges on transparency, robust support, and clear commitments. When procuring a medical mattress, decision-makers require comprehensive information on product lifecycle, maintenance, and post-purchase support. A reliable supplier provides clear documentation, including user manuals for proper handling and cleaning, and guidelines for optimal longevity. Furthermore, understanding the delivery timelines and logistic capabilities is crucial for seamless integration into operational schedules, particularly for large-scale procurements or urgent replacements.
Frequently Asked Questions (FAQs)
Q: How often should a pressure distribution mattress be cleaned?
A: The fluid-resistant and vapor-permeable cover of a Three-layer Pressure-Relief Mattress should be cleaned regularly with approved disinfectants after each patient use or as per facility infection control protocols. Refer to the specific product manual for detailed cleaning instructions and compatible cleaning agents to maintain the cover's integrity and anti-microbial properties.
Q: What is the expected lifespan of this pressure sore prevention mattress?
A: With proper care and maintenance, the foam core of a high-quality medical mattress typically has a warranty period of 5-7 years. The lifespan can vary based on usage intensity and adherence to cleaning and handling guidelines. Regular inspection for signs of wear, particularly foam compression or cover damage, is recommended to ensure continued efficacy.
Q: Are custom sizes available for specific bed frames?
A: Yes, leading manufacturers often offer customization options for the Three-layer Pressure-Relief Mattress to accommodate unique bed frame dimensions or specialized clinical needs. This includes variations in length, width, height, and specific cut-outs or zoning requirements. Contact our sales team with your specific requirements for a tailored solution.
Delivery and Warranty Commitments
Standard delivery for our Three-layer Pressure-Relief Mattress is typically within 2-4 weeks for major orders, with expedited options available for urgent requirements. Each medical mattress is backed by a robust warranty: a 7-year warranty on the foam core against manufacturing defects and loss of pressure relief properties, and a 2-year warranty on the cover fabric. Our commitment extends to comprehensive customer support, including technical assistance, maintenance guidance, and prompt resolution of any product-related inquiries, ensuring optimal performance and peace of mind for our healthcare partners.
Authoritative References and Clinical Insights
The design and efficacy of advanced pressure relief mattresses are grounded in extensive clinical research and adherence to global regulatory frameworks. The following references underscore the importance of evidence-based practice in selecting and utilizing these critical medical devices.
- European Pressure Ulcer Advisory Panel (EPUAP), National Pressure Injury Advisory Panel (NPIAP) and Pan Pacific Pressure Injury Alliance (PPPIA). Prevention and Treatment of Pressure Ulcers/Injuries: Clinical Practice Guideline. 3rd Edition. Emily Haesler (Ed.). EPUAP/NPIAP/PPPIA: 2019.
- International Organization for Standardization (ISO) 13485:2016. Medical devices — Quality management systems — Requirements for regulatory purposes.
- National Institute for Health and Care Excellence (NICE). Pressure ulcers: prevention and management. Clinical guideline [CG179]. 2014, last updated 2019.
- Food and Drug Administration (FDA). Medical Devices. Code of Federal Regulations, Title 21, Part 880 (General Hospital and Personal Use Devices).
- Cochrane Database of Systematic Reviews. Support surfaces for pressure ulcer prevention.
-
Mattresses Designed for Back Pain ReliefNewsAug.08,2025
-
Innovative Wave Mattresses for Ultimate ComfortNewsAug.08,2025
-
High-Quality Mattresses for Hospital BedsNewsAug.08,2025
-
High-Quality Mattresses for Every NeedNewsAug.08,2025
-
Healthcare Foam Mattress: Sleep Better, Heal FasterNewsAug.08,2025
-
Cube Mattress for Daily ComfortNewsAug.08,2025
-
How Hospital Mattress Choices Directly Impact Patient Comfort and CareNewsAug.05,2025

