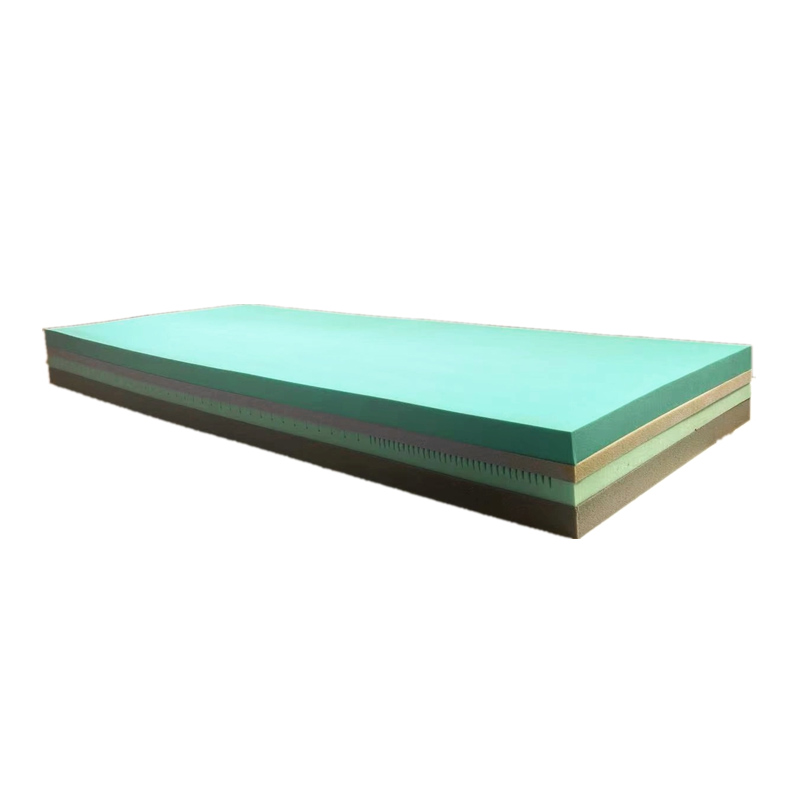
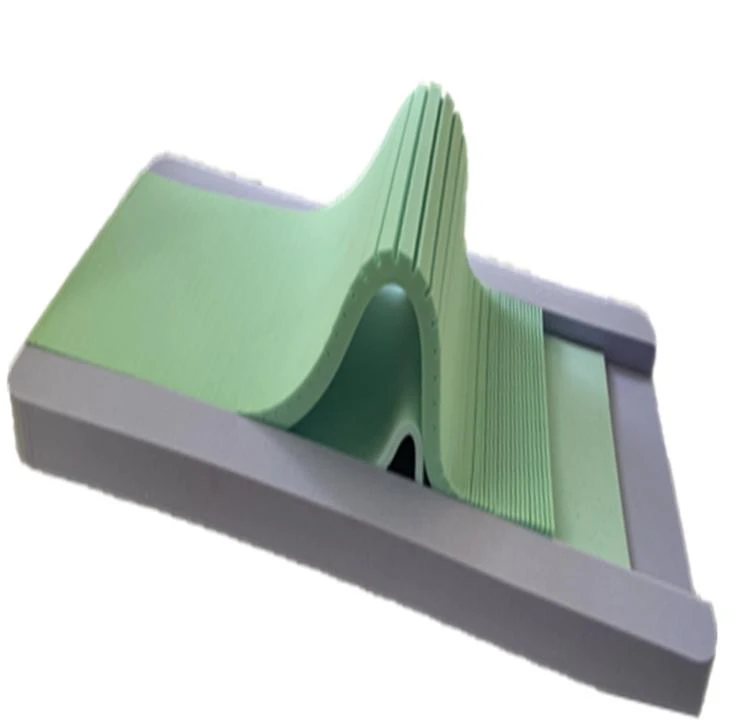
Product Presentation

Biocompatibility

Oriented for clinical application, invasive care can be performed directly on the mattress. The mattress has the characteristics of anti-infection, biocompatibility, durability and easy cleaning. It has passed the biological evaluation of ISO 10993 medical devices. The product has undergone stimulation and Skin sensitization test and in vitro cytotoxicity test verification.
Multi-layer design

The mattress adopts high grade polyurethane foam, which has been designed in multiple layers, taking into account the characteristics of pressure reduction and comfort. Its advantage is that it not only effectively prevents the occurrence of pressure bedsores, but also provides patients with a comfortable sleeping environment, which helps patients to rest better and restore immunity.
High garde polyurethane foam
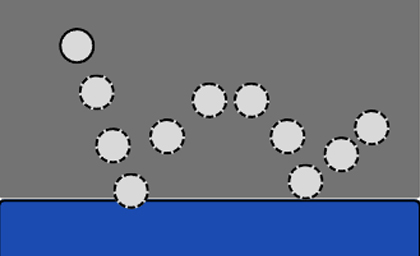
The unique polyurethane foam production scheme enables the mattress to achieve excellent comfort and resilience. By dropping the steel ball (resilience test), the steel ball is bounced with a high amplitude and softly, showing excellent mechanical performance. After a special incision design, it achieves the effect of wrapped decompression.
Clinical professionalism

Silicone cover for clinical care(Interventional therapy homologous material)
• Two-way High Elasticity: Pressure injury prevention PI-A1 - Reduces Shear Forces
• Waterproof, bodily fluid-proof, surface hydrophobic: Pressure Ulcer Prevention PI-B2 - Avoid Wet Environments
• Breathable, moisture-permeable, and active antibacterial, Gram group test active antibacterial rate > 95%: pressure ulcer prevention PI-B1 - creating an effective nursing microenvironment
• *PI pressure injury 《International Guidelines for the Prevention and Treatment of Pressure Injury》
Certifications

The mattress has outstanding functionality, and the inner core and outer cover have been tested and certified by independent organizations.
•Mattress inner core has no formaldehyde volatilization
•Flame retardant for both outer cover and the inner core
•The raw materials of the product have OEKO-TEX100 textile certification, which meets the requirements of the ecological environment for human contact
Product Attribute
Standard sizes:1950*900*160mm (+/-5mm) Specifications can be customized
Mattress weight:14kilogram
Load bearing:150kilogram
- 1.Mattress core:
•Polyether and polyester mixed high-grade polyurethane foam with a soft and delicate nest-like foam structure.
•Multi-layer design, a variety of materials with different hardness and resilience cooperate with each other, with a ventilation hole design in the middle, which can flexibly match the body curve, give weight distribution, pressure redistribution, and body parts with different weights can form an effective support Promote blood circulation and effectively prevent pressure sores.
•Excellent physical and mechanical properties. Hardness, rebound rate, and tear strength are all higher than national standards.
- 2.Cover:
•The outer cover is two-way elastic, waterproof, anti-blood-borne pathogens, anti-mildew and anti-bacterial, breathable and moisture-permeable, flame-retardant, anti-disinfection, long life, and no danger of infection.
•The outer cover has a hidden zipper design, which is easy to disassemble , can be washed, can be dried at low temperature, and can be wiped and disinfected with 75% medical alcohol or ≤1000ppm sodium hypochlorite solution.
- 3.With bio-compatibility and clinical application orientation, invasive care can be performed directly on the mattress. The mattress has the characteristics of anti-infection, bio-compatibility, durability and easy cleaning, and has passed the biological evaluation of ISO 10993 medical devices. The product has been verified by irritation and skin sensitization tests and in vitro cytotoxicity tests.
- 4.Suitable for patients with a variety of clinical pressure ulcer risks, sleep more comfortably, safe and noiseless.
- 5.The product warranty period is one year, the expected service life is five to eight years, and the maintenance response time does not exceed 48 hours.
- 6.Product-related test reports
- Polyurethane foam (cotton core) mechanical performance appraisal report
- Polyurethane foam (cotton core) flame retardant test report
- Polyurethane foam (cotton core) environmental protection report (formaldehyde)
- ▲Biological Evaluation of Medical Devices - Irritation and Skin Sensitization Test Report
- ▲Biological Evaluation of Medical Devices - In Vitro Cytotoxicity Test Report
- Wear-resistant and anti-fatigue test report of outer cover
- Cover flame retardant test report
- Cover antibacterial test report
- Medical ICU series mattress pressure sensor test report