Fév . 10, 2025 10:29
Back to list
Gynecological examination table mattress
Navigating the complex world of medical equipment can be daunting, especially when considering the regulatory certifications required for compliance and safety. CE certification is one of the crucial validations needed for medical bed positions in the European Union. This badge not only signifies safety and adherence to health standards but also opens doors to a broad marketplace.
Beyond technicalities, authoritativeness is reinforced by collaborating with reputable third-party organizations for product testing. These organizations conduct exhaustive tests that validate the product's performance claims. Their affiliation not only strengthens the credibility but also assures healthcare providers and patients of the bed’s reliability. The partnership with well-recognized institutions acts as an endorsement, backing the manufacturer’s claims with impartial verification. Trustworthiness, an often understated element, finds its foundation in transparency and post-certification activities. Manufacturers must ensure that the certification process and product specifications are transparent, providing full disclosure of materials and functionalities. Continued compliance through rigorous internal audits and open lines for customer feedback further instills trust. Moreover, manufacturers must implement a robust post-market surveillance system to track the product’s performance in real-world conditions. This proactive approach helps in tweaking and upgrading the bed designs as necessary, guaranteeing a safe and effective product lifecycle. It's evident that achieving CE certification for medical bed positions is not merely a regulatory formality, but an integral part of ensuring product excellence. This certification process enhances the functionality and safety of medical beds, ultimately improving patient care. The pursuit of CE certification is as much about conforming to legal standards as it is about embracing quality and innovation. It aligns the goals of manufacturers with the needs of the healthcare industry, fostering an environment of continuous improvement and patient satisfaction. In conclusion, the process of obtaining CE certification for medical beds is comprehensive and multifaceted, demanding a blend of experience, expertise, authoritative partnerships, and trust. Once achieved, it unlocks potential in the competitive European market while reinforcing the commitment of manufacturers to deliver high-quality, safe, and reliable products. This rigorous adherence not only complies with regulatory demands but also elevates the standing of manufacturers as pioneers in medical innovation.
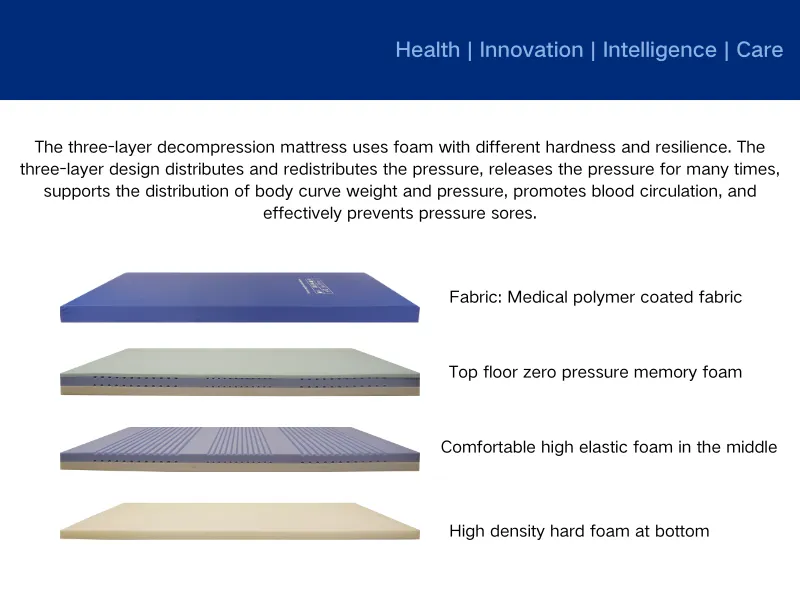

Beyond technicalities, authoritativeness is reinforced by collaborating with reputable third-party organizations for product testing. These organizations conduct exhaustive tests that validate the product's performance claims. Their affiliation not only strengthens the credibility but also assures healthcare providers and patients of the bed’s reliability. The partnership with well-recognized institutions acts as an endorsement, backing the manufacturer’s claims with impartial verification. Trustworthiness, an often understated element, finds its foundation in transparency and post-certification activities. Manufacturers must ensure that the certification process and product specifications are transparent, providing full disclosure of materials and functionalities. Continued compliance through rigorous internal audits and open lines for customer feedback further instills trust. Moreover, manufacturers must implement a robust post-market surveillance system to track the product’s performance in real-world conditions. This proactive approach helps in tweaking and upgrading the bed designs as necessary, guaranteeing a safe and effective product lifecycle. It's evident that achieving CE certification for medical bed positions is not merely a regulatory formality, but an integral part of ensuring product excellence. This certification process enhances the functionality and safety of medical beds, ultimately improving patient care. The pursuit of CE certification is as much about conforming to legal standards as it is about embracing quality and innovation. It aligns the goals of manufacturers with the needs of the healthcare industry, fostering an environment of continuous improvement and patient satisfaction. In conclusion, the process of obtaining CE certification for medical beds is comprehensive and multifaceted, demanding a blend of experience, expertise, authoritative partnerships, and trust. Once achieved, it unlocks potential in the competitive European market while reinforcing the commitment of manufacturers to deliver high-quality, safe, and reliable products. This rigorous adherence not only complies with regulatory demands but also elevates the standing of manufacturers as pioneers in medical innovation.
Share:
Latest news
-
How Hospital Mattress Choices Directly Impact Patient Comfort and CareNewsAug.05,2025
-
Hospital Mattress Topper Compatibility IssuesNewsAug.05,2025
-
Choosing the Best Hospital Mattress for Home UseNewsAug.05,2025
-
Blue Hospital Mattress Material Safety StandardsNewsAug.05,2025
-
Best Firm Hospital Bed Mattress for Elderly: A Wholesaler’s Guide to Quality Care SolutionsNewsAug.05,2025
-
Adjustable Bed Compatibility with Hospital MattressesNewsAug.05,2025
-
Sleep Tracking Mattress GuideNewsJul.28,2025

